



What is “SaaS+”?
The EU requires that every single drug package be counterfeit-proof and traceable to the manufacturer. For this purpose, each package is provided with an individually traceable serial number and a barcode. Due to many legal regulations and high investment equipment, serialisation is very costly for a manufacturer and special know-how is also required. Together with its partners, MSK offers serialisation as a flexible service that you can integrate seamlessly into your logistics chain. We call this ” Serialisation as a service” or “Saas+”.
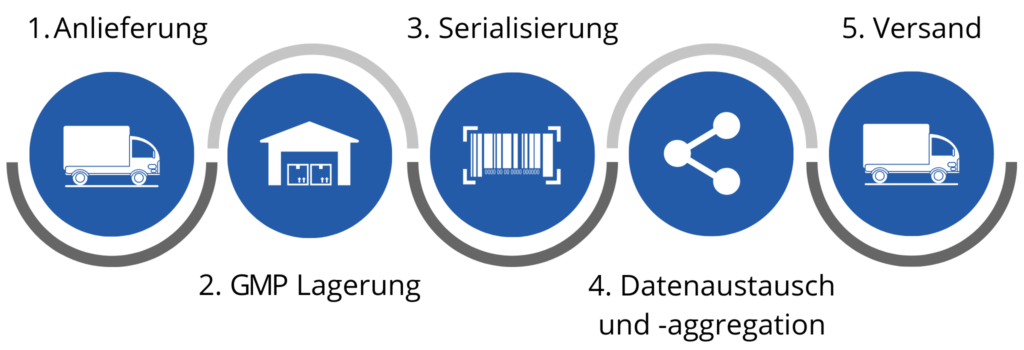
How “Serialisation as a service” works
Your products are delivered to our GDP and GMP certified storage facility in Heppenheim. This is conveniently located in the Rhine-Neckar metropolitan region. This is where the packaging is serialized. Our machines are so flexible that we can handle all package sizes and quantities. The data is entered into the European Medicines Verification System via secure systems and in accordance with the European data protection regulations, so that it is possible to check at any time whether this medication is genuine and where it comes from. The products are then reintegrated into your supply chain and further distributed. In this way, the regulations of the EU Pharmaceutical Directive are fulfilled without great effort. In addition, you can order different quantities according to changing forecasts and pay only per piece and without fixed costs.

What advantages does SaaS+ offer?

100% SCALABLE
We serve all quantities, as we have exclusive access to equipment through our partners. And if your forecast changes next month, we will adapt to it.

COST-EFFECTIVE
Instead of procuring the high-investment equipment for serialisation yourself and hiring and training your own personnel, you benefit from our economies of scale.

VARIABLE COSTS
We do not charge you any fixed fees. All costs are per serialized package, so you only have variable costs.

GMP CERTIFIED
As experts in pharmaceutical logistics, all storage facilities and processes at MSK Pharmalogistik are of course GDP and GMP certified.
Get a non-binding consultation now and find out how SaaS+ can help your company.
Was sagen unsere Kunden über “Serialisierung as a Service”?
Klosterfrau GmbH
For the storage and distribution of healthcare products, we have an extensive logistics infrastructure with 4,500 m² of storage space, which was built especially and exclusively for the strict requirements of the pharmaceutical industry.
Togal Werk AG
For the storage and distribution of healthcare products, we have an extensive logistics infrastructure with 4,500 m² of storage space, which was built especially and exclusively for the strict requirements of the pharmaceutical industry.
Pille XYZ Co KG
For the storage and distribution of healthcare products, we have an extensive logistics infrastructure with 4,500 m² of storage space, which was built especially and exclusively for the strict requirements of the pharmaceutical industry.
Wilder Füße GmbH
For the storage and distribution of healthcare products, we have an extensive logistics infrastructure with 4,500 m² of storage space, which was built especially and exclusively for the strict requirements of the pharmaceutical industry.
The SaaS+ Team

MSK Pharmalogistic GmbH is the pharmaceutical industry’s full-service provider for logistics, contract packaging and services in the fields of personnel, sales and marketing.

Metacarp GmbH offers innovative ERP solutions compliant with GMP for the healthcare industry. The features include production, quality assurance, batch tracing and immediately usable software for serialization.

Tracekey solutions GmbH is an expert for compact and flexible serialisation projects in the manufacturing industry. Their IT solutions focus on high transparency for the entire product life cycle.

With its modular packaging and supply chain solutions, Laetus GmbH is the industry leader for inline quality control and is primarily responsible for the development of Secure Track & Trace Solutions (S‑TTS).
Frequently Asked Questions about SaaS+
You have full control. We download the desired serial numbers from your Level‑4 system and transmit them again after production.
We use different methods to seal the package. Whether with an in-line labeler for Tamper Evident stickers or by manual application of hot glue. If desired, we also use your provided labels.
Before the first order can be produced by us, the process must be coordinated in advance and you must be integrated into our system environment. This is necessary only once and is product independent.
As a rule, machines can only print certain sizes. By using our own serialisation labeling machine, we are able to serialize all package sizes.
We can carry out serialisation according to the EU directive, but also Russia or UDI for you. Other forms of serialisation can of course be included after the request has been submitted.

Get a non-binding consultation now and find out how SaaS+ can help your company.
Terms & Conditions Privacy Policy © 2019, MSK Pharmalogistic GmbH. All rights reserved.

