Pharma Packaging and Co-Packing – GMP-Certified and Flexible
Our expertise covers packaging, repackaging, kitting, assembly, and serialization of medical devices, pharmaceuticals, dietary supplements, and cosmetics. As a GMP-certified co-packing service provider with ISO 13485 certification, we guarantee the highest quality – from contract manufacturing and repackaging to market release. With MSK, you save valuable resources and can focus on your core business.
Trusted Packaging. Proven Responsibility.
Regulatory requirements in pharmaceutical packaging present significant challenges. As your reliable partner, we stand for innovative and tailored solutions that allow you to focus fully on what matters most.
Daniel Richter — Head of Manufacturing


Pharmaceutical Product Assembly – Flexible and GMP-Compliant
MSK offers more than logistics – we take care of the precise processing and packaging of pharmaceutical and cosmetic-related products. Our services in assembly, packaging, and kitting are tailored to your specific requirements and guarantee maximum flexibility – even for short-term changes. Whether co-packing, repackaging, or visual inspection: as an experienced partner along the healthcare value chain, we stand for quality, efficiency, and absolute reliability.
What we offer in pharmaceutical packaging:
- Repackaging & filling
- New packaging
- 100% visual inspections
- Serialization and printing
- Bundling, shrinking, and sleeving
Kitting and Co-Packing – Customized and Regulatory-Compliant
We bring together what belongs together. As a co-packing service provider for pharmaceutical companies, we realize your projects in kitting, co-packing, and build-to-order with the highest precision. To help you optimize your value chain, we offer maximum flexibility and extensive expertise in pharmaceutical packaging. If required, we also support you with material sourcing and regulatory matters. Leverage our experience as a true competitive advantage for your business.
Our kitting and co-packing services at a glance:
- End-to-end packaging consulting
- Procurement and QC testing of packaging materials
- Customized forecast planning
- Market release in accordance with Annex 16

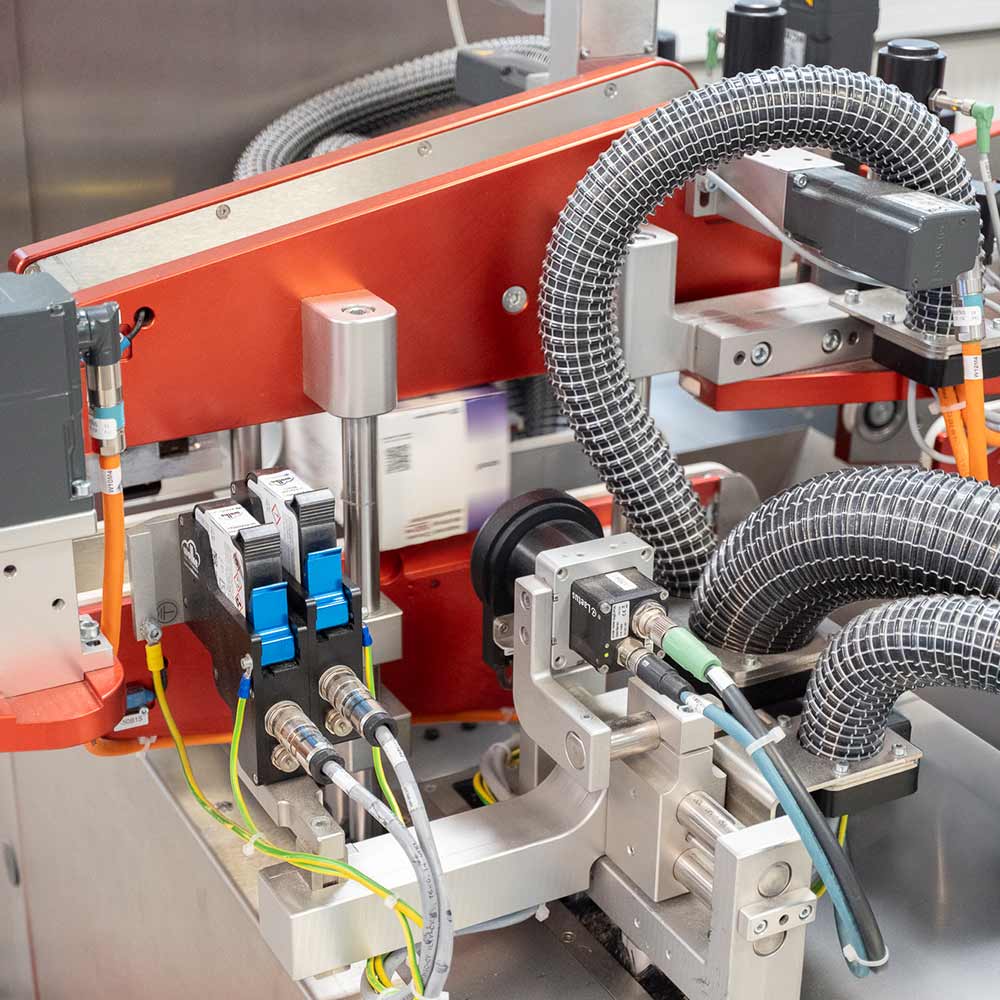
Serialization in the Pharmaceutical Industry – Systematic Protection Against Counterfeiting
Since the introduction of EU Directive 2011/62/EU in 2019, we have established ourselves as an expert in the serialization of pharmaceutical products. In addition to serialization in accordance with EU FMD / FDA requirements, we also provide a wide range of services related to product protection and anti-counterfeiting.
Our Serialization-as-a-Service offering includes:
- Serialization in compliance with EU FMD / FDA
- Aggregation
- Bundle serialization
- Decommissioning
- Level 4 management
- Upload management to the EMVO Hub
How can we support your pharma packaging and co-packing needs?







